Drug discovery
Work with us to identify and advance novel approaches with the potential to deliver the next generation of cancer medicines.
Review articles on synthetic chemistry aren’t the usual output of medicinal chemists. Yet a team of Cancer Research Horizons medicinal chemists have come to write a review on the hydroxylation of steroids, detailing discoveries in the beautiful and complicatedly chiral world of steroid chemistry.
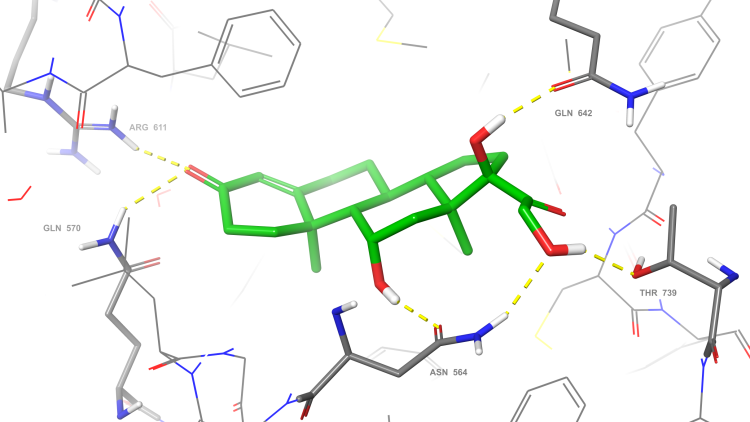
This exciting output from Cancer Research Horizons’ medicinal chemists highlights the adaptability, scientific excellence, and fearlessness in face of complexity that we build our reputation on. The review1 is an impressively detailed and comprehensive work, more customary from established experts in the synthesis field than drug discovery scientists. For Cancer Research Horizons it has confirmed that expert synthetic understanding and review of the literature is an enabling building block for medicinal chemistry and should be seen as an integral part of the design of molecules. This blog details the journey of how the review came about: from the historical contribution of the Institute of Cancer Research (ICR) to the field of steroidal oncology treatments, to Cancer Research Horizons’ recently developed expertise in the beautiful and complicatedly chiral world of steroid chemistry.
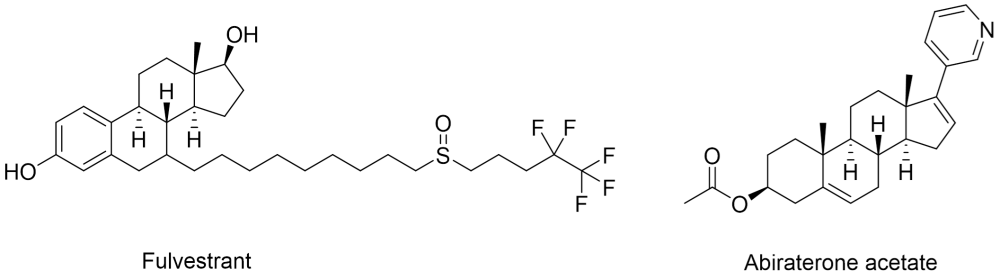
Drugs with steroidal cores are not new in oncology treatments. Fulvestrant, which is used to treat HR-positive breast cancer, contains an oestrogen-like core, which binds to the oestrogen receptor and causes instability in the protein, resulting in degradation2. Reporting clinical potential in the early 90s, it was miles ahead of the wave of recent interest in targeted protein degradation. Due to its high clearance and poor bioavailability3 it is dosed intramuscularly. Another example, abiraterone acetate, inhibits cytochrome P45017α to prevent testosterone production, and is dosed orally for the treatment of prostate cancer. Abiraterone acetate was developed in the 90s by Michael Jarman and chemists at the ICR in London and is outlined in the Cancer Research UK blog here alongside the description of the medicinal chemistry research4.
For a long time, medicinal chemists have discussed how to “escape [the] flat-land”5 of aromatic rings that is so often the starting point for medicinal chemistry. Containing numerous stereocentres and copious vectors for substitution, steroids are highly three-dimensional structures, making them a structurally interesting place to begin drug discovery. So, when we were considering a steroidal starting point, there was some excitement, and more than a little apprehension around the previously unencountered territory of steroidal chemistry and its associated challenges. Following the success found by the ICR chemists with abiraterone acetate, the Cancer Research Horizons team felt inspired to proceed.
One of the key properties of steroids is that they are highly lipophilic, allowing them to be permeable to cell membranes, though detrimental to other properties. In medicinal chemistry, alongside trying to escape the poor solubility that comes with the flat aromatic structures alluded to earlier, a common struggle is to reduce clearance and poor solubility driven by lipophilicity. Knowing that the most prevalent functional group seen in marketed drugs is a hydroxyl moiety6, and observing that is especially common with steroidal drugs, we sought to modulate drug properties through addition of hydroxy functionality, which reduces lipophilicity (measured by log P) by an average of 0.8 units per −OH group7.
Exploration of how to attempt this chemistry led Cancer Research Horizons’ chemists to learn extensively about how to hydroxylate steroids with different regio- and stereoselectivity. This knowledge is now consolidated and shared in the review, in the hope that it is a valuable resource for the wider scientific community.
The full review, now published in Chemistry: A European Journal, details the incredible work over the last two decades in this field. We have also come a long way in their understanding of steroidal chemistry, recruiting experts in biocatalysis (Lucy Harwood*) and total synthesis (Hossay Abas) to support experienced medicinal chemists Pete Blencowe and me as well as the rest of the chemistry team, which catalysed the authorship of this review.
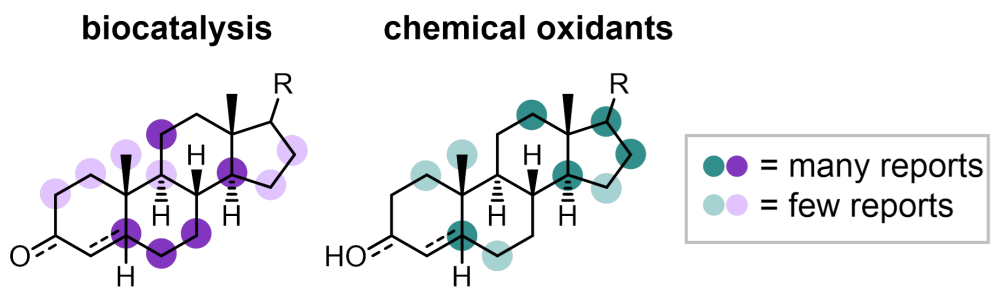
The review focuses on the three main methods for the hydroxylation of steroidal C(sp3)–H bonds: biocatalysis, metal-catalysed C–H hydroxylation, and organic oxidants, such as dioxiranes and oxaziridines. It highlights reactions that install a hydroxyl group to a variety of different steroidal substrates with a high degree of regio-, chemo- and stereoselectivity. We hope that the review is both interesting and informative, and that it will help make some of these chemistries more accessible for future chemistry projects.
Cancer Research Horizons’ bold drug discovery model embraces risk, enabling us to work on more complicated chemistries and starting points, which many medicinal chemists would have avoided. The complexity of the molecules and the chemistry covered in the review highlights the tenacity, resilience, and expertise that the chemistry department possesses. The department has a wide range of synthetic capabilities and equipment, including the recent introduction of photochemical and electrochemical synthesis alongside the standard techniques. The chemistry team have used reviews of the literature alongside innovative approaches to develop new routes to previously inaccessible targets, showing a fearlessness in the face of complicated chemistry. We hope to publish some of these advances soon so keep an eye out!
*Current role: Senior scientist in biocatalysis at AstraZeneca, Gothenburg.